Localized Proteomics of the Endometrium: Pilot Study of IVF Biopsies using Laser Capture Microdissection and Mass Spectrometry
Introduction
Significance
Proper endometrial function is key to a healthy pregnancy. Misregulation of endometrial growth, as in luteal phase defect (LPD), is associated with recurrent miscarriage in about 10% of cases (Potdar & Konje, 2005). Endometriosis is characterized by faulty estrogen-dependent regulation of the endometrial lining (Lim & Wang, 2010). One study found that, in pregnant women suffering from advanced endometriosis, there was around a 1.5-fold higher risk of infant congenital malformation when compared against controls. The data highlights the importance of studying the molecular mechanisms of endometrial regulation to elucidate clinical methods by which we can improve pregnancy outcomes for patients suffering from endometrial complications (Liang et al., 2019).
Localized proteomics is a targeted way to study these mechanisms. Characterizing the proteome of various endometrial sub-tissue structures allows understanding of protein location, which allows detailed inference into protein function. Because the endometrium metabolically and physically supports the developing conceptus, and endometrial pathologies are associated with poor pregnancy outcomes, developing a deep understanding of localized protein function is imperative to progression in this field. For example, using a localized proteomic approach, we can create protein-targeted therapies for various endometrial pathologies, thus improving birth outcomes.
Before delving into the molecular mechanisms of endometrial function, however, it is important to understand how and why the endometrium is regulated.
Background
Human female reproduction involves two cycles: the uterine and ovarian cycles.
The uterine cycle regulates endometrial thickness. Hormone-regulated fluctuations in thickness, characteristic of the monthly menstrual cycle, are important to provide an ideal environment for embryonic implantation into the endometrium. Implantation is necessary to ensure physical security for the embryo. Early in pregnancy, endometrial glandular secretions metabolically support the conceptus while the main nutritive organ, the placenta, is still in development. The anatomy of the endometrium facilitates its secretory function. The crypts are the sites of secretion discharge. The glands feed into the crypts via exocytosis and membrane fusion. Additionally, in mice, it is well-established that implantation occurs in the crypts. However, controversy still exists in the case of human implantation.
The ovarian cycle describes the growth and maturation of the egg cell. Once the egg is released from its “shell” (the follicle) into the oviduct, it is ready for fertilization by a sperm cell. This “ready stage” of the egg coincides with the time when the endometrium is at its thickest. In this way, the female reproductive system optimizes egg receptivity to sperm with endometrial thickness, in order to have an optimal environment for a developing conceptus.
In vitro fertilization (IVF) is an assistive reproductive technology that artificially controls these reproductive cycles via administration of exogenous hormones and subsequent in vitro fertilization.
Image of IVF insemination in vitro.
Prior to the fertilization step, however, the receptivity of the patient’s endometrium to hormones must be assessed. After all, if unresponsive, the endometrium may shed during pregnancy. Additionally, the implanted embryo will not receive adequate metabolic support from endometrial secretions if the endometrial layer is too thin. To test for receptivity, a biopsy of the endometrium is taken. These biopsies were used in this pilot study.
Study Aim
This article aims to characterize the proteome of endometrial biopsies and propose potential roles of the discovered proteins in endometrial function. The study discussed in this article was a pilot study to test the compatibility of HypoThermosol FRS Preservation Solution (Sigma-Aldrich; reference: H4416) for biopsy preservation, with laser capture and mass spectrometry. However, being a pilot, this study did not localize tissue structures. Rather, whole tissue sections were pulled down and analyzed. As such, the next step of this study is to separately characterize the proteomes of different tissue structures to obtain more thorough results.
Materials and Methods
Endometrial biopsy samples (n=3) arrived frozen in Optimal Cutting Medium (OCT). We dissolved the OCT with Ca²⁺ and Mg²⁺-free PBS (saline) and re-froze the samples in an OCT mold compatible with cryosectioning.
We sliced the samples with a cryostat at a 10 micron thickness and placed them on Director slides. We dyed the slides with 0.01% toluidine blue and desiccated samples using a lyophilizer. We isolated protein using laser microdissection, dropping cuts into LMD buffer (RapiGest to denature and solubilize protein, TCEP to reduce disulfide bonds, TEAB to buffer pH, and MS-SAFE to prevent non-trypsin protein digestion). Finally, we analyzed the sample proteome using mass spectrometry. Visit this post to learn more about laser capture microdissection and preparatory procedures.
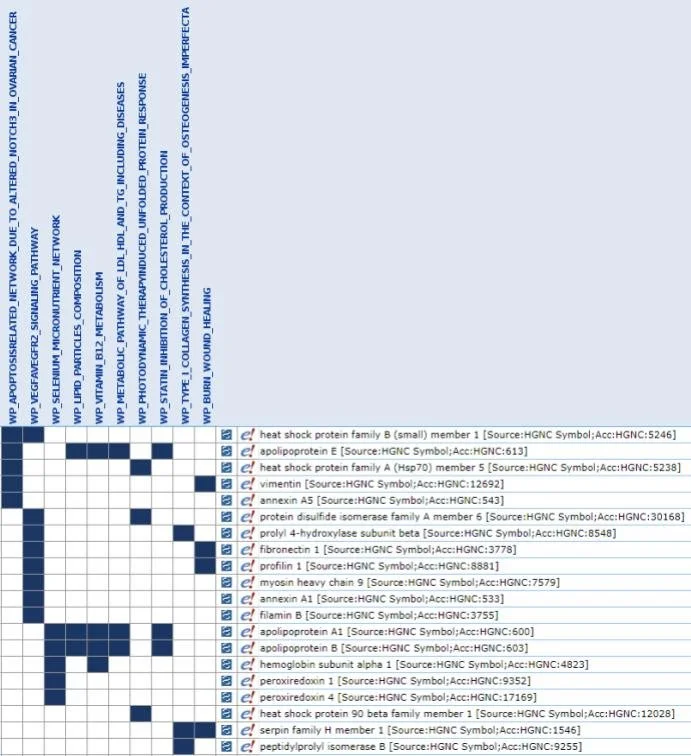
Mass spectrometry results were analyzed using Gene Set Enrichment Analysis (GSEA) to determine processes and pathways in which the detected proteins could have a role in. The WikiPathway and Reactome databases were used for this analysis. To learn more about GSEA, visit this post.
Results
The proteins found from mass spectrometry are involved in a variety of pathways. Following is a discussion of the potential roles of these protein pathways in endometrial function. Specific functions explored include: endometrial cycling (including immune activity), nutrition, and glandular supply (including protein modification & arterial blood supply).
Discussion
Endometrial Cycling
Endometrial cycling is integral to the optimization of endometrial thickness for implantation. As such, it is expected to find proteins regulating senescence and apoptosis of endometrial cells, which aid in timing menstruation.
Because apoptosis and senescence are processes that occur in all cells, and involve precise regulation of various parts of the cell, analyses directly from the databases would not yield helpful information, since the proteins will be involved in many processes. So, upon analysis of the GSEA hits, which placed emphasis on the processes rather than the proteins and took k/K values into account, we noticed that the Apoptosis-related network due to altered Notch3 in ovarian cancer and Glycolysis in senescence processes were significantly present in the tissue. From this, we deduced that the function of these proteins is to regulate cellular metabolism and lifespan in the monthly uterine cycle, which provides optimal implantation ground for the embryo.
Immune Activity
We observed an overwhelming immune signature in the endometrial samples. The following proteins were labeled by the GSEA software as exclusively of immune function, and were found in both databases: complement C3, HSP 90 alpha family class B member 1, GST pi 1, transthyretin, gelsolin, lactotransferrin, pyruvate kinase M1/2, peroxiredoxin 4, myosin heavy chain 9, and vitronectin.
The reason for an immune signature in the endometrium may lie in the process of endometrial cycling. Because the GSEA software labeled the proteins as either involved in neutrophil degranulation or innate immunity (or both), we can hypothesize that the granzymes and perforins released from neutrophil degranulation processes cause cell death of decidual and endometrial cells, thus triggering decidual sloughing.
Nutrition
Endometrial secretions are integral for embryonic nutrition prior to establishment of the placenta. As such, we see many lipid carrier proteins in the results, including apolipoproteins A1*, B, and E from both the WikiPathway and Reactome datasets.
The presence of lipid carriers suggests a nutritive function of endometrial secretions. Lipids, being energy-dense molecules, would be ideal for efficient nutrition for the embryo. Thus, the proteome of the endometrial tissue suggests a nutritive function, which is consistent with what is observed prior to placental establishment.
*Although apolipoprotein A1 is a common blood protein, it can still be considered a significant result because of the presence of other less common apolipoproteins in the same area. Regardless of whether or not the presence of A1 is a coincidence, apolipoprotein presence in the endometrium cannot be denied.
Glandular Supply
Protein Modification
Because glandular secretions are vital to embryonic nutrition, glandular activity of the endometrium is very high. As such, protein modification apparatus is necessary.
Consistent with this idea, we see many heat shock proteins, which are involved in chaperoning protein folding, in our data from both databases. In particular, we see HSP family B member 1, HSP family A member 5, and HSP 90 beta family member 1 in both WikiPathway and Reactome datasets. In addition to these, the Reactome dataset includes HSP family A member 8 and HSP 90 alpha family class B member 1. The prevalence of heat shock proteins and knowledge of their general function led to the idea that protein modification apparatus is needed in the endometrium to keep up with modifying the massive amounts of protein being secreted by the glands.
Arterial Blood Supply
In such a glandular tissue, adequate vascularization is needed to supply glands with enough material to filter out and secrete. Proliferation of blood vessels is controlled by many mechanisms; here, I explore two pathways (RAS and Notch) that appeared to have significance in our data.
From the Reactome database, we found the RAB1A and RAB1B proteins of the member RAS oncogene family in the tissue. The RAS oncogene family produces proteins that increase vascularization. In the context of cancer, overly promoted vascularization facilitates metastasis, or the movement of malignant tumors from one part of the body to another via blood vessels. In the context of the endometrium, however, increased vessel proliferation is beneficial to provide glands with a steady blood supply for filtration and secretion.
From the WikiPathway database, we found proteins that were involved in the Apoptosis-related network due to altered Notch3 in ovarian cancer pathway. The Notch pathway is involved in vascular proliferation, differentiation, and function (Hunkapiller et al., 2011; Gridley, 2010). As such, the presence of Notch pathway proteins in endometrial tissue alludes to the importance of vascular proliferation in the area.
Conclusion
Because this pilot study did not isolate protein by anatomical structure, and instead gathered all protein from biopsies, we don’t have a concrete idea about the function of proteins in the endometrial tissue. As such, there is a study underway focusing on the proteome of sub-tissue structures to elucidate protein function in the endometrium.
Studying the endometrial proteome accounting for sub-tissue location is integral to the advancement of knowledge about endometrial function and the development of protein-targeted therapies for endometrial pathologies.
References
Gridley T. (2010). Notch signaling in the vasculature. Current topics in developmental biology, 92, 277–309. https://doi.org/10.1016/S0070-2153(10)92009-7.
Hunkapiller, N. M., Gasperowicz, M., Kapidzic, M., Plaks, V., Maltepe, E., Kitajewski, J., Cross, J. C., & Fisher, S. J. (2011). A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development (Cambridge, England), 138(14), 2987–2998. https://doi.org/10.1242/dev.066589.
Liang, Z., Yin, M., Ma, M., Wang, Y., & Kuang, Y. (2019). Effect of Maternal Advanced Endometriosis on Risk of Congenital Malformations for Infants Born After in vitro Fertilization and Frozen-Thawed Embryo Transfer: Analysis of 28,600 Newborns. Frontiers in endocrinology, 10, 763. https://doi.org/10.3389/fendo.2019.00763.
Lim, H. J., & Wang, H. (2010). Uterine disorders and pregnancy complications: insights from mouse models. The Journal of clinical investigation, 120(4), 1004–1015. https://doi.org/10.1172/JCI41210.
Potdar, N., & Konje, J. C. (2005). The endocrinological basis of recurrent miscarriages. Current opinion in obstetrics & gynecology, 17(4), 424–428. https://doi.org/10.1097/01.gco.0000175363.20094.bd.