Laser Capture Microdissection (LCM) Procedure & Medical Applications
Laser capture microdissection (LCM) is a laboratory technique that uses a laser to precisely isolate tissue structures for analysis, allowing inference about cellular activity. This information aids in the development of diagnostic tools, facilitating early treatment and better patient outcomes.
LCM Procedure
SAMPLE PREP
Before conducting microdissection, one must make the tissue of interest LCM-compatible via desiccation. LCM uses a laser to isolate tissue sections, and any liquid water present will cause refraction of the laser. This diminishes cutting precision. Therefore, one must sufficiently dehydrate the tissue to prevent any such imprecisions.
Additionally, simple glass slides are not adequate to hold tissue for LCM. Because the microdissection scope relies on gravity to drop dissected tissue into collection wells, we use specialized Director slides manually coated with poly-D-lysine to enhance sample adhesion (Hembrough, 2012). When the laser contacts this coated slide, the energy of the laser is transferred to the aptly-named energy transfer matrix on the slide. As a result, the matrix vaporizes and tissue is dropped into the collection well (“The cutting edge”, 2007).
Many methods are used to dessicate samples for LCM. My favorite is lyophilization. Frozen tissue samples are cryosectioned* and placed on Director slides. After removal of embedding medium (such as Optimal Cutting Medium), the tissue is stained to enhance visualization such as with toluidine blue. The slides are subsequently placed in a lyophilizer, which sublimes the water in the tissue samples. By using sublimation and bypassing the liquid water phase, we eliminate the possibility of retaining liquid water in the tissue sample, thus preserving the precision of laser capture.
Other sample preparation methods include the following:
The samples can be fixed in acetone (dehydrant). The sample is then stained, treated with xylene (which makes the histology clearer) (Gubler, 2019), and further dehydrated in alcohol (which replaces water in the tissue) (Turashvili et al., 2007). One disadvantage of this method is that acetone’s strong lipid-solvent properties may cause cellular embrittling (Rolls, n.d.).
The samples can be treated with a graded ethanol series (which replaces water in the tissue) and rapidly dried with compressed nitrogen (Gormley et al., 2021).
Among the methods presented above, lyophilization is my favorite because of the range of tissues for which anatomy can be preserved. Even naturally “wet” tissue types, such as that of the endometrium, are compatible with this method because water is smoothly removed (unlike in other methods, where dehydration occurs in a stepwise fashion), preventing major damage to the histology. However, tissue quality rapidly deteriorates following lyophilization. Therefore, microdissection should be performed on samples as soon as possible following lyophilization to provide optimal results. “Dry” tissues exist in relatively dry conditions (within the appropriate range for biological tissues, of course). Because there isn’t much water to begin with, any desiccation method can preserve histology fairly well.
*My mentor (Matthew Gormley, Fisher Lab, UCSF) experimentally determined that the optimal cutting thickness for endometrial tissue is 14 microns. Optimal thickness varies around tissue type and preparation technique, but 14 microns is still a good estimate for many tissues. To yield the most material from laser capture, one must cut tissue at the maximum thickness for which anatomy can still be preserved.
MACHINERY + SOFTWARE
The most common system used for LCM is the Leica Microdissection Microscope and associated software. The scope and software are extremely user-friendly; with the help of my mentor, I learned the basic maneuvers in a day or two, and was autonomous with the scope by day three.
In addition to standard microscope parts (eyepieces, objective lenses, focus knobs, stage motion knobs, etc.), this scope includes internal mirrors (which direct the laser to specific locations for cutting), a fiber optic cable (which guides light to form a laser) (Landry, 2020), and an internal well-insert attachment point (which collects dissected samples).
Image of the Leica LCM microscope. Source.
One convenient feature of the software is the “Create Fast Overview” function. This creates a map of your slide so you can quickly jump between tissue sections and easily keep track of which ones you have already microdissected.
The screenshot on the left shows the location of the “Create Fast Overview” button in the Leica LCM microscope menu. The image on the right is a picture of the map of the slide that the program created. The purple sections are toluidine blue-stained endometrial biopsies taken from IVF patients for proteomic analysis.
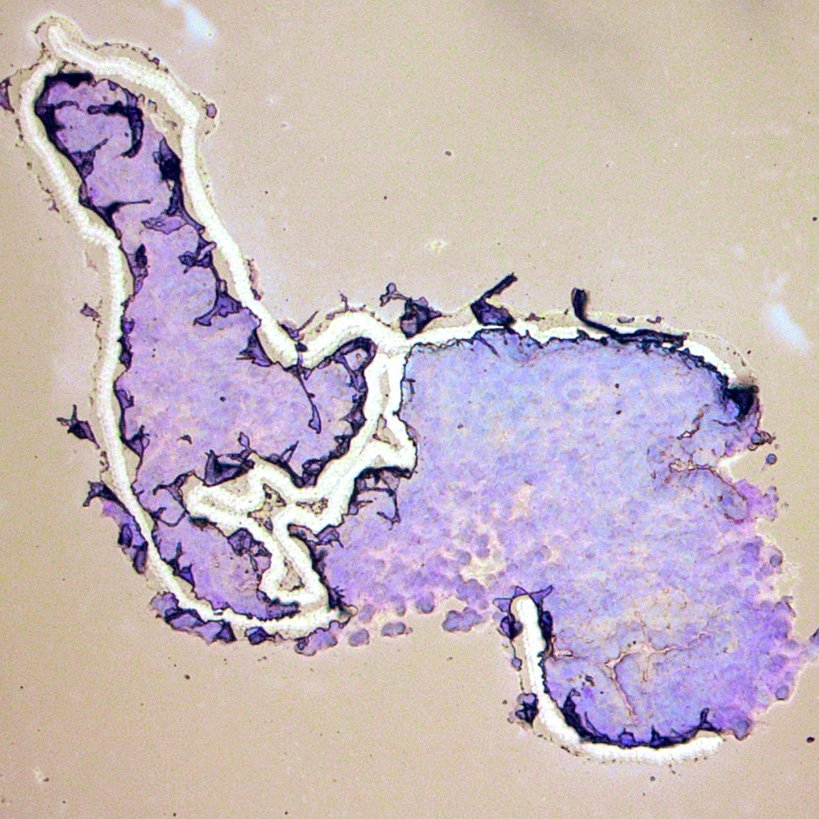
Below are some pictures I took while dissecting endometrial tissue samples.
At 10x magnification, this is what the endometrial sections look like. The first image was captured prior to any microdissection. The second image was captured after the epithelium was removed. The third image was captured after crypt removal. To learn more about the endometrium, visit this post. One important thing to notice from these images is the white space in the dissected areas. These areas are where the energy transfer matrix of the Director slide has been ejected.
Why LCM is a Big Deal
Prior to the development of laser-based technologies, microdissection was extremely challenging. Scientists used a fine needle attached to a micromanipulator, and, with great dexterity, sectioned out parts of tissue (Murray, 2007). Because of the great dexterity required of this method, other cell isolation techniques (such as flow cytometry) were more commonly used to achieve a similar goal.
Unlike these techniques, however, laser capture microdissection allows for precise isolation of cellular structures. Their small size relative to other tissue components makes isolation by other methods very challenging. LCM allows precise molecular profiling of various sub-tissue regions, while avoiding the issue of sample contamination from more predominant structures (Craven & Banks, 2001).
In histopathological studies, observing the molecular changes of disease at the sub-tissue structural level allows us to gather a more detailed report of disease-specific localized changes in gene expression. The ultra-precise and localized analyses, made possible by laser capture, aid in the development of biomarker-based tools to identify early-stage pathology (Fend & Raffeld, 2000).
Now that laser capture is feasible, you may ask why we haven’t gotten rid of other cell isolation techniques altogether. After all, with LCM, we know exactly where in the tissue that cells come from. However, one must think about the goal of the procedure. If the objective is to sort cells by stark morphological differences and analyze activity, flow cytometry is the way to go. If the objective is to observe a cellular drug response in vivo, a tumor xenograft analysis might be best (“3 steps,” 2019). Even though all the techniques mentioned above allow us to observe cellular activity in some way, each technique has its own procedural differences that make it more suited to one experiment over another. In short, because different experiments have different goals, different procedures may be necessary to achieve optimal results within a particular experiment (Curran et al., 2000). Examples of experiments for which LCM was optimal will be explored in the next section. In general, LCM will yield high-quality results for any study requiring precise isolation of low-abundance tissue structures.
EXAMPLES OF LCM USE IN LITERATURE
LCM is useful for molecular characterization of sub-tissue structures. Localized profiling of diseased vs. normal tissue allows for the development of diagnostic tools. Examples of LCM-assisted advances follow.
Preeclampsia: novel insights from global RNA profiling of trophoblast subpopulations. LCM was used to isolate three different kinds of trophoblasts. RNA analysis allowed characterization of the transcriptome for placental tissue from normal or severely preeclamptic patients. Transcriptome comparison yielded a short list of dysregulated molecules in diseased tissue, thus providing a potential avenue towards a diagnostic biomarker for early preeclampsia detection (Gormley et al., 2017).
Laser microdissection and two-dimensional difference gel electrophoresis reveal proteomic intra-tumor heterogeneity in colorectal cancer. LCM was used to isolate different kinds of tissue from colorectal tumors. Samples were analyzed using mass spectrometry. It was discovered that different parts of tumors expressed certain proteins more intensely than in the same parts in noncancerous tissue. The proteomic difference points to a potential colorectal cancer diagnostic test targeting the proteins upregulated in malignant tissue (Sugihara et al., 2013).
Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. LCM was used to differentiate between the gene expression patterns of invasive ductal and lobular carcinomas. Tissue structures were microdissected to yield localized data regarding differences in gene expression between the two types of breast cancer. Certain proteins were observed to be differentially regulated relative to their other-group counterparts. Using this data, a protein-based diagnostic method specifically tailored to each type of breast cancer can be developed (Turashvili et al., 2007).
Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. LCM was used on lupus patients’ renal tissue to isolate two types of immune cells that participate in the development of lupus-associated kidney disease. Cytokine balances for each immune cell group were assessed, and it was discovered that cytokine profile corresponds to renal pathology subclassification, thus opening an avenue for specific diagnoses of systemic lupus erythematosus (Wang et al., 2010).
Future Applications of LCM
The development of biomarker-based diagnostic tools relies on identifying differences between diseased and non-diseased tissue. Laser capture microdissection allows sections of tissue to be isolated for molecular profiling. Therefore, laser capture is an integral technique for the future of diagnostic medicine.
Biomarker-based diagnostics are important to develop. Because of their sensitivity, they allow for early detection of disease. Examples of diseases where diagnostics are needed include preeclampsia, many cancers, autoimmune disorders, and other conditions. In these diseases, early detection is beneficial because timely intervention majorly improves patient outcomes.
Laboratories that have adopted laser capture are making huge advances in our understanding of disease processes. LCM is the future of medicine.
References
Craven, R. A., & Banks, R. E. (2001). Laser capture microdissection and proteomics: Possibilities and limitation. PROTEOMICS, 1(10), 1200–1204. https://doi.org/10.1002/1615-9861(200110)1:10<1200::aid-prot1200>3.0.co;2-q
Curran, S., McKay, J. A., McLeod, H. L., & Murray, G. I. (2000). Laser capture microscopy. Molecular Pathology, 53(2), 64–68. https://doi.org/https://doi.org/10.1136%2Fmp.53.2.64
Fend, F., & Raffeld, M. (2000). Laser capture microdissection in pathology. Journal of Clinical Pathology, 53(9), 666–672. https://doi.org/10.1136/jcp.53.9.666
Gormley, M., Oliverio, O., Kapidzic, M., Ona, K., Hall, S., & Fisher, S. J. (2021). RNA profiling of laser microdissected human trophoblast subtypes at mid-gestation reveals a role for cannabinoid signaling in invasion. Development, 148(20). https://doi.org/10.1242/dev.199626
Gormley, M., Ona, K., Kapidzic, M., Garrido-Gomez, T., Zdravkovic, T., & Fisher, S. J. (2017). Preeclampsia: Novel insights from global RNA profiling of trophoblast subpopulations. American Journal of Obstetrics and Gynecology, 217(2). https://doi.org/10.1016/j.ajog.2017.03.017
Gubler, M. (2019). Xylene Substitutes. Fixation on Histology Blog. Retrieved July 28, 2022, from https://www.nsh.org/blogs/michael-gubler/2019/07/16/xylene-substitutes
L., S. (2019, July 15). 3 steps for successful tumor xenograft analysis. PerkinElmer. Retrieved July 28, 2022, from https://www.cisbio.eu/content/3-steps-for-successful-tumor-xenograft-analysis/
Landry, J. (2020, November 26). Fiber Lasers: Everything You Need To Know. Laserax. Retrieved July 28, 2022, from https://www.laserax.com/blog/fiber-laser
Murray, G. I. (2007). An overview of laser microdissection technologies. Acta Histochemica, 109(3), 171–176. https://doi.org/10.1016/j.acthis.2007.02.001
Rolls, G. (n.d.). Fixation and Fixatives – Fixing Agents Other than the Common Aldehydes. Leica Biosystems. Retrieved July 28, 2022, from https://www.leicabiosystems.com/us/knowledge-pathway/fixation-and-fixatives-3-fixing-agents-other-than-the-common-aldehydes/
Sugihara, Y., Taniguchi, H., Kushima, R., Tsuda, H., Kubota, D., Ichikawa, H., Fujita, S., & Kondo, T. (2013). Laser microdissection and two-dimensional difference gel electrophoresis reveal proteomic intra-tumor heterogeneity in colorectal cancer. Journal of Proteomics, 78, 134–147. https://doi.org/10.1016/j.jprot.2012.11.009
Turashvili, G., Bouchal, J., Baumforth, K., Wei, W., Dziechciarkova, M., Ehrmann, J., Klein, J., Fridman, E., Skarda, J., Srovnal, J., Hajduch, M., Murray, P., & Kolar, Z. (2007). Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer, 7(1). https://doi.org/10.1186/1471-2407-7-55
Wang, Y., Ito, S., Chino, Y., Goto, D., Matsumoto, I., Murata, H., Tsutsumi, A., Hayashi, T., Uchida, K., Usui, J., Yamagata, K., & Sumida, T. (2009). Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clinical and Experimental Immunology, 159(1), 1–10. https://doi.org/10.1111/j.1365-2249.2009.04031.x